Measuring carbon dioxide can be tricky but important to monitor during the winemaking and bottling process.
The level of dissolved carbon dioxide (CO2) in wine has a significant sensory impact. In general, CO2 enhances freshness and acidity perceptions, decreases sweetness, intensifies bitterness and astringency and can lead to prickliness. Depending on the wine, too little CO2 can make white wines flat and too much can make reds harsh and tannic.
It is therefore very important for winemakers to manage CO2 in order to guarantee the right concentrations in their wines. However, one needs to remember that it is always difficult to manage gas in wine especially when (like in the case of CO2) the measuring devices are neither very accurate nor easy to use. People are also quite unsure on how CO2 can be protected when wine is bottled.

CO2 in wine
CO2 is a gas naturally produced by yeast during fermentation and as all gases, has the ability to dissolve into liquids. CO2 is more soluble in wine than oxygen (around 40 times more). The solubility of a gas in a specific liquid is measured as the saturation concentration. Henry’s law is used to quantify the solubility of gases. The solubility of a gas in a liquid is directly proportional to the partial pressure of that gas above the liquid. This relationship is written as:
Pco = Hco ·C where Pco is the partial pressure of oxygen, C is the concentration, and Hco is the solubility constant, also known as Henry’s constant.
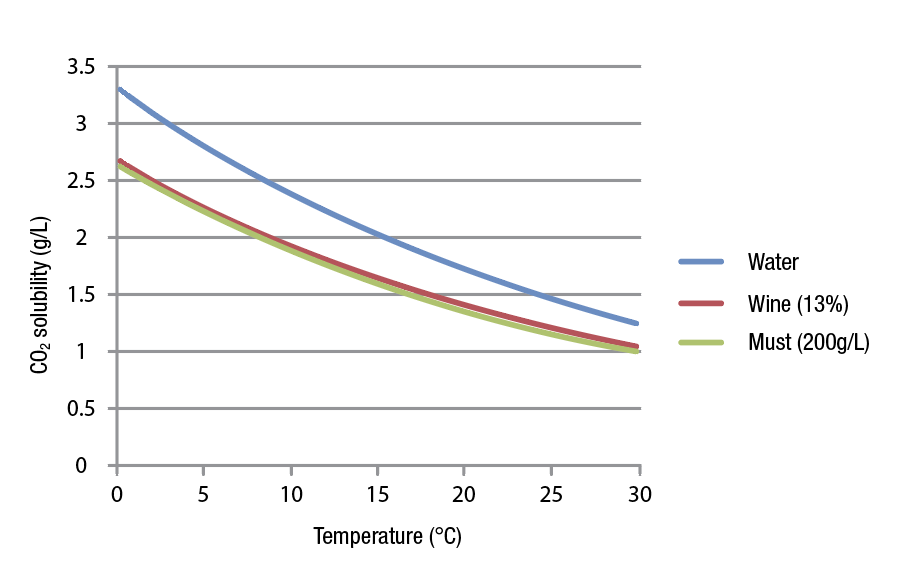
This key parameter is affected by temperature and composition of the liquid medium. Therefore, the amount of CO2 that will be present in the liquid at a given pressure (for example atmospheric pressure) will change depending on the temperature and the type of liquid. Temperature has a big effect on the solubility. When temperature rises, CO2 becomes less soluble. Alcohol and sugars content have also an impact, by decreasing the amount of CO2 being dissolved (Figure 1).
CO2 can also be hydrated by water to form carbonic acid (H2CO3), which is a weak acid, but the reaction is in favour of the CO2 form and, because of the wine pH range, the H2CO3 formed is not being able to dissociate in hydrogenocarbonate (HCO3-).
As air contains a small amount of CO2 (~0.03%), the CO2 content in wine tends to decrease over time during the winemaking process but also during storage until an equilibrium with air is reached (around 0.5mg/L of dissolved CO2 in the wine).
Measuring CO2 in wine
Measuring the CO2 dissolved in wine remains challenging, although some methods have been developed.
The OIV (International Organisation of Vine and Wine) proposes two methods to measure CO2 in wine.
The first one is based on pH titration. The sample of wine is fixed to pH 10 – 11 and then titrated with a standard acid solution. The carbon dioxide content is calculated from the volume of acid needed to change the pH from pH 8.6 to 4, which corresponds to the area in which CO2 is converted from the bicarbonate to carbonic acid form. A degassed blank is done the same way to take the titration of other acids present in the wine into account and correct the results accordingly.
The second method is based on pressure release and monitoring. Sodium hydroxide is added in the sample to bind the CO2. The wine is put in a flask connected to a manometer and the CO2 is released with sulfuric acid. The resulting pressure increase is then measured and compared to a calibration curve.
Unfortunately, these two reference methods are only suitable for laboratories and are not really accessible for wineries.
In wineries, the “carbodoser”, a cheap and easy-to-use device, is probably the most widely spread. Wine (100 mL) sampled in a graduated cylinder is shaken to degas the CO2. The shrunken wine volume and temperature are then measured and a handy chart translates the result into milligrams per litre. The main drawback of this method is its poor accuracy (+/- 100 mg/L) which is too low to guarantee good CO2 management in wineries.
Another common measurement technique is the Pressure/Temperature method. A specific volume of wine is entrapped in a chamber and the system forces the CO2 equilibrium between the wine and the air. Thanks to the higher solubility of CO2 compared to those of O2 and N2, CO2 will increase the total pressure measured, allowing the CO2 measurement.
Another technique on the market is the thermal conductivity (TC). In fact thermal conductivity of CO2 is much lower than the one of O2 and N2, and can be measured by creating a 1°C difference between two surfaces. This measurement has to be done in a gas phase, so a specific diffusion membrane separates the wine from the TC detector. Nitrogen is used to purge the detector between two measurements. Calibration has to be carried out very often.
As a conclusion, we can say that the wine industry is still after an affordable technology that would allow for accurate CO2 measurement in an easy set-up.
CO2 in the bottle
Before bottling, the amount of CO2 dissolved in wine is adjusted, but bottling and then bottle ageing will affect CO2 concentration.
During bottling, the wine is moved from the tank into the bottle, which can potentially involve contact with air and therefore loss of CO2.
The filling of the bottle is the main step where CO2 will escape from wine during bottling, with greater losses occurring at beginning and end of the bottling run, as in the case of oxygen. Bottles coming out early can potentially have less CO2 due to mixing of the wine with the air present in the piping and filter, resulting in CO2 migration from the wine into the air. At the same time, the last volumes of wines being bottled are also exposed to higher CO2 loss, as this is the wine that remains in the tank longer and therefore more in contact with air.
So, if no care is taken, the first and last hundred bottles (according to the size of the line) of the process will contain more oxygen and less CO2. To limit this phenomenon, the use of inert gas is necessary. Especially in whites and rosés, the use of a 50% CO2/50%N2 mixed gas is a good solution.
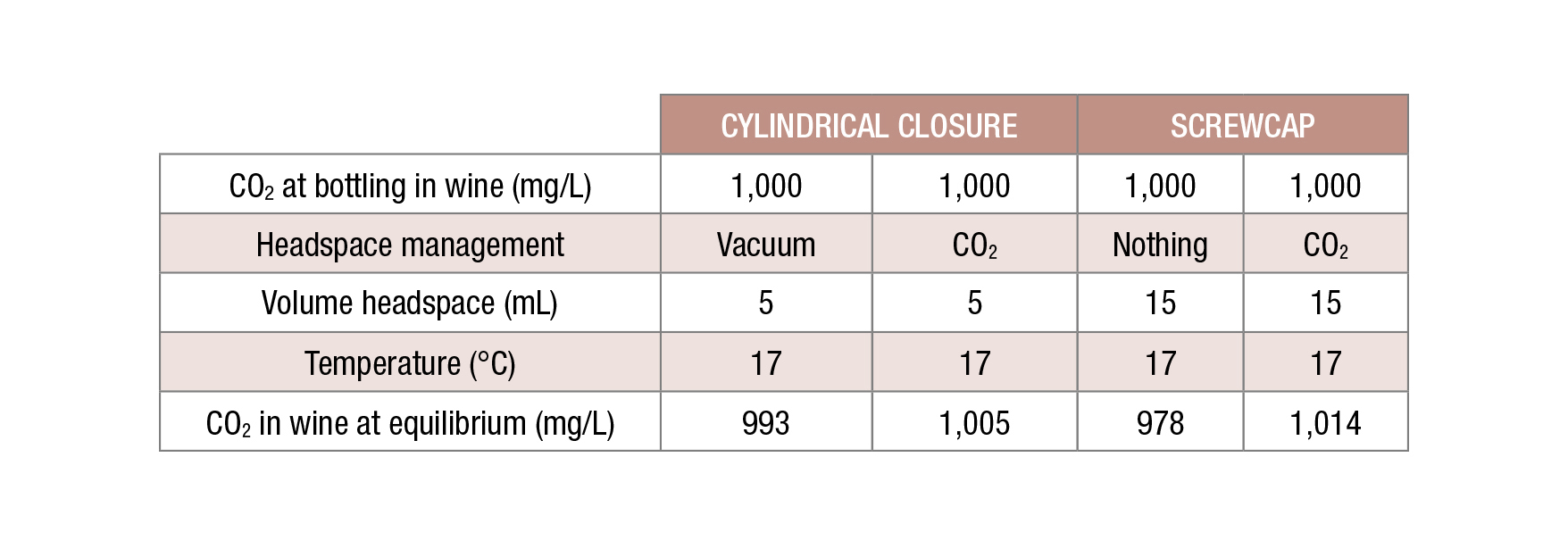
After bottling, CO2 pressures will reach an equilibrium between dissolved and headspace. If no CO2 is used to inert the headspace, some CO2 from the wine will migrate to the headspace. As the headspace represents a small volume, the decrease of dissolved CO2 remains rather small. This decrease accounts for 1% in a case of an inner closure and 2% for a screw cap (Figure 2). If the headspace is inerted with CO2, diffusion of CO2 from the headspace into the wine could occur, but the increase of dissolved CO2 due to this process is somewhat negligible.
During bottle storage, due to the higher partial pressure of CO2 in the bottle compared to ambient air, loss of CO2 over time is commonly observed. This loss will be regulated by the closure through its specific permeability. As in the case of oxygen permeability (OTR), closures have a carbon dioxide permeability, which can be expressed as carbon dioxide transmission rate (CO2TR).
Temperature can affect the CO2 loss over time. The CO2TR is dependent on the CO2 gradient, which is, in this case, driven by the CO2 pressure in the bottle. A lower temperature increases the CO2 solubility in the wine and so decreases the CO2 pressure in the bottle. As a consequence, at lower temperature, loss of CO2 will be slower.
Conclusion
Managing CO2 in wine is key to wine quality but remains very challenging. In fact the challenges are linked to the lack of easy-to-use and accurate methods to measure CO2 in wineries when winemakers have to make quick decisions while preparing the wines for bottling. If the right CO2 target can be reached before bottling, then good bottling practices and the closure choice can impact the level of consistency from one bottle to another.





